- Sorry, this product cannot be purchased.
-
Maternal Serum II Control, Level 3
Assayed, liquid human serum based control for maternal quadruple markers (AFP, hCG, Free Estriol and Inhibin A); Level 3 (6 x 2.5 mL)
-
MONO test (20 tests/package)
The Clearview® MONO test is a rapid chromatographic immunoassay used to detect infectious mononucleosis heterophile antibodies in whole blood, serum and plasma to aid in diagnosis of infectious mononucleosis. Infectious mononucleosis (IM) can resemble more serious infections that demand urgent treatment, so it is important to quickly and accurately diagnosis.
-
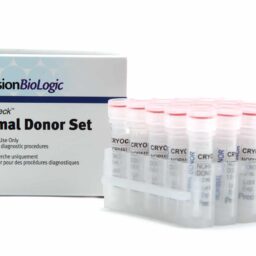
Normal Donor Sets (25 x 1.0 mL)
Controls & Specialty Plasmas
CRYOcheckâ„¢ Normal Donor Sets are manufactured from carefully screened male and female plasmas and sold in sets of 25 individual 1.0 mL aliquots.
- Platelet-poor
- Donors are screened negative for lupus anticoagulants
- Source plasmas are screened negative for all FDA-required tests
- 50 unique donors available on request
-
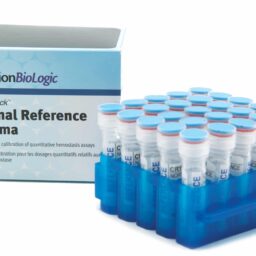
Normal Reference Plasma
Controls & Specialty Plasmas
CRYOcheckâ„¢ Normal Reference Plasma is intended for use as an assayed calibrator for all parameters listed below. Reference values are assigned by an independent, internationally-recognized reference laboratory, using international reference standards (where available). Assigned value, unit, and method used are indicated on the Assay Certificate provided with each lot number.
- 8-hour stability once thawed if refrigerated at 2 to 8 °C in the original capped vial
- Source plasmas are screened negative for all FDA-required tests
The following assays may be calibrated with CRYOcheck Normal Reference Plasma:
- Fibrinogen
- Factor II
- Factor V
- Factor VII
- Factor VIII:C
- Factor IX
- Factor X
- Factor XI
- Factor XII
- Factor XIII
- Prekallikrein
- vWF: Antigen
- vWF: Ristocetin Cofactor
- Antithrombin Activity
- Antithrombin Antigen
- Plasmin Inhibitor
- Plasminogen
- Protein C Activity
- Protein C Antigen
- Protein S Activity
- Protein S Free
- Protein S Total
-
pH Standard Buffer with Calibration, Blue, pH 10; 4 L
$175.27NIST-traceable reference materials include a calibration report from an ISO 17034 and ISO 17025 accredited laboratory- Choose from clear or color-coded standards from common calibration points
- 4 L (128 fl.oz.) capacity
- Calibrate at pH 10
- Easily identify blue color-coded formula
-
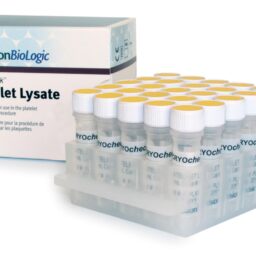
Platelet Lysate (25 x 1.0 mL)
CRYOcheck™ Platelet Lysate is a source of phospholipids intended for use in the platelet neutralization procedure (PNP) used to detect lupus anticoagulant (LA). Platelets are thoroughly washed to remove other cellular and protein contaminants and held at a concentration of 250,000 to 300,000 platelets/µL, then lysed by a freeze/thaw cycle.
- The frozen format eliminates reconstitution errors
- Replaces in-house collection and washing of platelets for the PNP
- 8-hour stability once thawed if refrigerated at 2 °C to 8 °C in the original capped vial
-
Pneumocystis jirovecii PCR Kit 1.0
The RealStar® Pneumocystis jirovecii PCR Kit 1.0 is an in vitro diagnostic test, based on real-time PCR technology, for the detection and quantification of Pneumocystis jirovecii specific DNA.
-
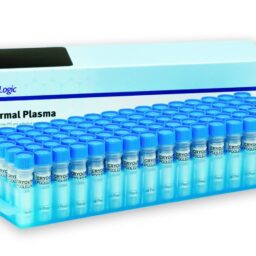
Pooled Normal Plasma
Controls & Specialty Plasmas
CRYOcheck™ Pooled Normal Plasma is comprised of platelet-poor plasma from 20 or more carefully screened male and female donors aged 18 to 66. It is recommended as a normal control for the one‑stage prothrombin time (PT) and activated partial thromboplastin time (APTT) assays. It may also be used as an alternative to laboratory-collected pools of normal plasma.
- Each lot number is verified to be normal for factors II, V, VII, VIII, IX, X, XI, and XII (factor assay value sheet available upon request) as well as fibrinogen
- Donors are screened negative for lupus anticoagulants
- Source plasmas are screened negative for all FDA-required tests
-
Reference Control Normal
Controls & Specialty Plasmas
CRYOcheckâ„¢ Reference Control Normal is intended for use as an assayed normal control for parameters listed on the Assay Certificate. Reference values are assigned by an independent, internationally-recognized reference laboratory, using international reference standards (where available).
- 8-hour stability once thawed if refrigerated at 2 °C to 8 °C in the original capped vial
- Source plasmas are screened negative for all FDA-required tests
-
Restraint Belt, 1-Piece
$80.42Brand: FERNO Canada
- For Stair Chair.
Additional Information:
- Colour:Â Black
- Length:Â 7′
-
RSV Card (22 tests/package)
The Clearview® MONO test is a rapid chromatographic immunoassay used to detect infectious mononucleosis heterophile antibodies in whole blood, serum and plasma to aid in diagnosis of infectious mononucleosis. Infectious mononucleosis (IM) can resemble more serious infections that demand urgent treatment, so it is important to quickly and accurately diagnosis.
-
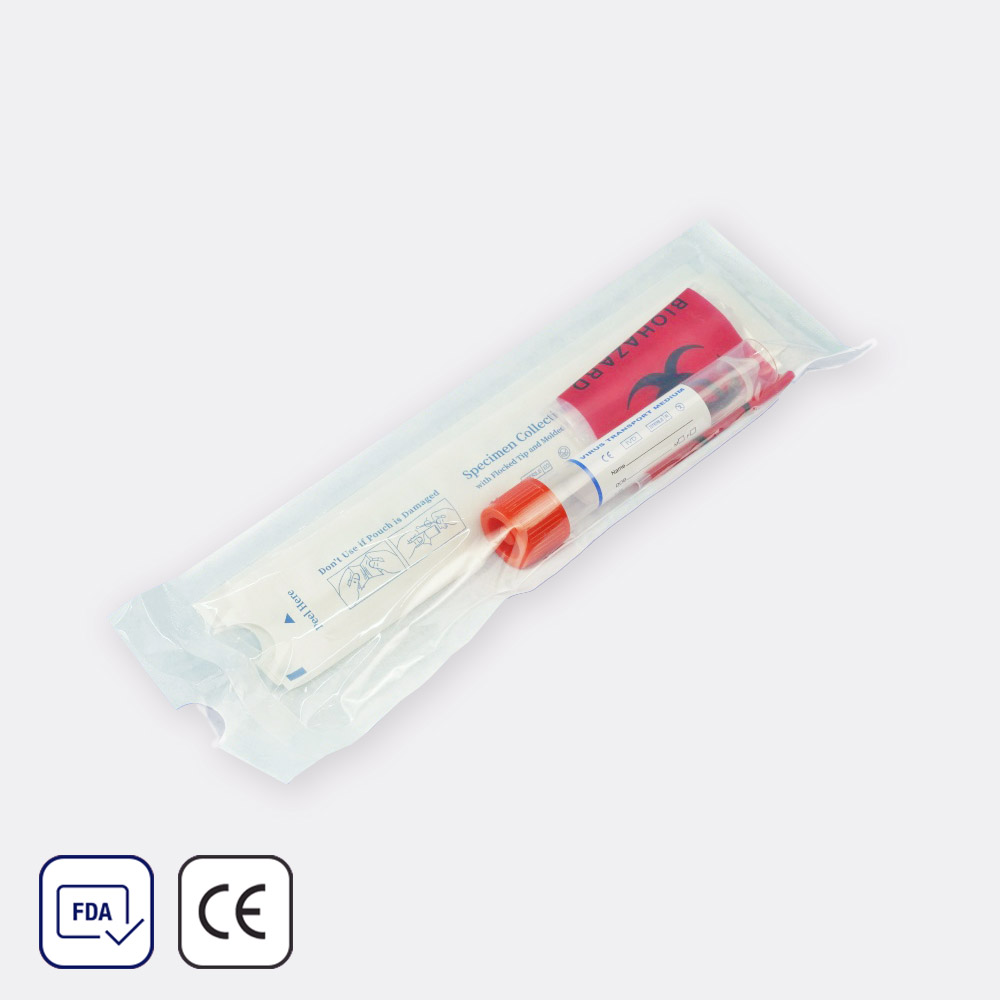
Specimen Collector
$140.07Brand: Vernacare
Ideal for collecting and measuring urine samples.
- Biodegradable
- Bleach-free
- Graduated
- Material: Pulp paper
- Disposable
Additional Information:
- Volume: 700mL
- Brand: Mid-Stream®
-
Virus Sampling Kit with 2 Swabs (20-pack)
Classification∶ IVD
Shelf Life∶ 2 years
Purpose∶ Virus specimen collectionFeatures:
- Quality Flocked Swab with Molded Breakpoint
- Superior Collection Capacity and Sample Elution
- Universal Room Temperature System of Keeping
- High-Quality Tube and Cap Which is Harmless for Virus
- Stable Transport Meidum which guarantee testing result
-
Weak Lupus Positive Control
Controls & Specialty Plasmas
CRYOcheck™ Weak Lupus Positive Control is intended for use as a positive control in assays for lupus anticoagulant (LA). It contains citrated human plasma collected from donors that have tested positive in accordance with the revised criteria of the SSC Subcommittee for the Standardization of Lupus Anticoagulants. CRYOcheck Weak Lupus Positive Control is a weak positive LA control that challenges an assay’s sensitivity to LA, allowing users to be confident that weak LA positive patient samples will be accurately detected.
- 8-hour stability once thawed if refrigerated at 2 °C to 8 °C in the original capped vial
- Source plasmas are screened negative for all FDA-required tests
-
ZeptoMetrix Flu/RSV/SARS-CoV-2 Negative Control
$264.55Qualitative Molecular Control
NATtrol™ products are ready-to-use, inactivated full process controls designed to evaluate the performance of molecular tests. They can be used to verify assays, train laboratory personnel, and monitor assay-kit lot performance. NATtrol™ products contain intact organisms and should be run in a manner similar to clinical specimens.
-
ZeptoMetrix Flu/RSV/SARS-CoV-2 Positive Control
$301.60Qualitative Molecular Control
NATtrol™ products are ready-to-use, inactivated full process controls designed to evaluate the performance of molecular tests. They can be used to verify assays, train laboratory personnel, and monitor assay-kit lot performance. NATtrol™ products contain intact organisms and should be run in a manner similar to clinical specimens.